Today, nuclear energy provides 21% of the UK’s electricity. That’s an important big chunk of our energy mix. Let’s look at the science behind nuclear energy.
How It Works: Nuclear Power Station shows you how nuclear fission leads to electricity.
Chain Reaction
Nuclear fission reactions split uranium atoms apart, releasing a great deal of energy.
In nuclear fission, there’s not just one atom that splits, but a whole bunch of them. Like a chain reaction, once one atom splits, it goes on to split more atoms.
Here is a simple video courtesy of Glasgow Science Centre to show the idea of nuclear fission as a chain reaction, using mousetraps and ping pong balls!
Missing Mass = Energy
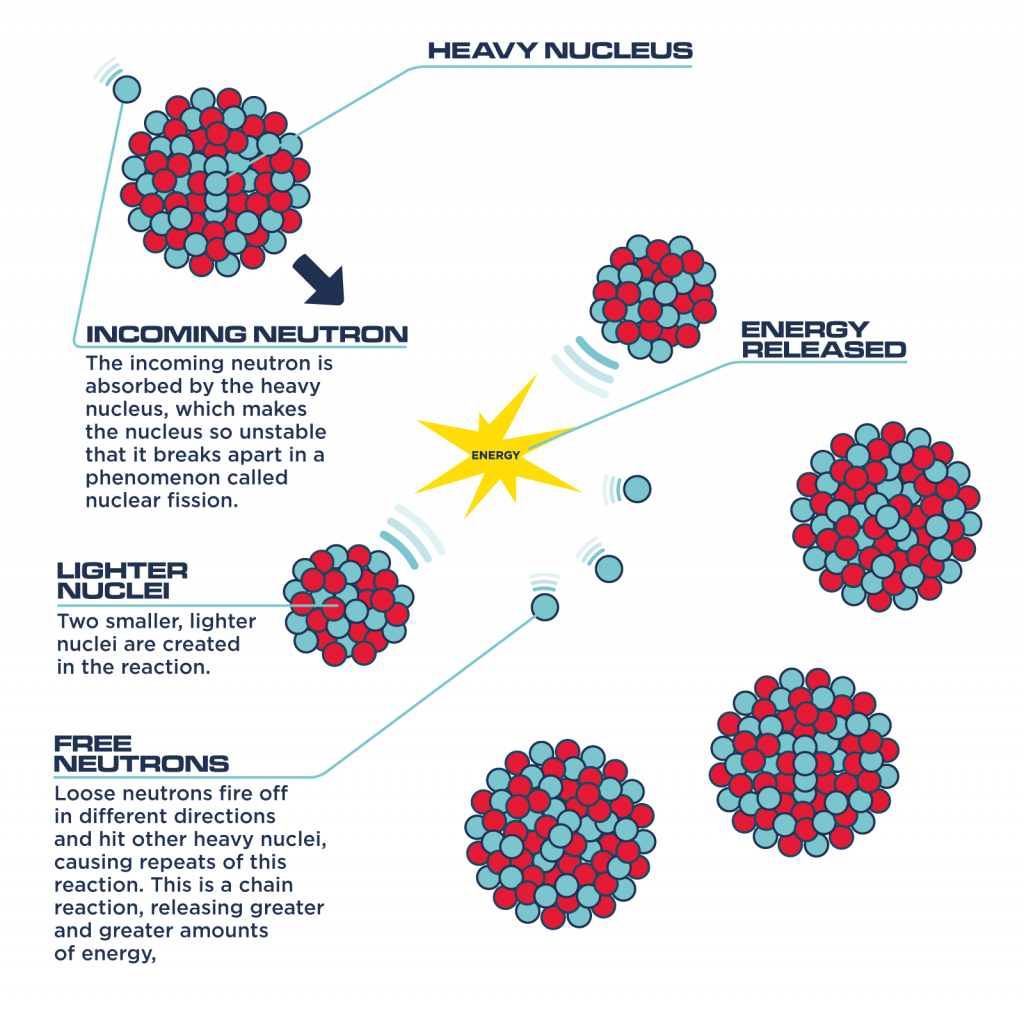
Once the atom is split, what is leftover does not equal the same amount of mass (m) that the atom had before; some mass is lost.
Think of it like cutting a slice of cake into two smaller slices. You would expect squishing those two new smaller slices together would be the same as the old slice. What if lots of crumbs fell away as you were cutting the cake? Squishing the two new slices together wouldn’t quite make the old slice. There would be something missing.
This missing mass (or crumbs!), is what makes nuclear power possible.
You probably know the famous equation and scientist that discovered where the missing mass went.
E = mc2
E = energy
m = mass
c = speed of light
Yup! The one and only Albert Einstein discovered that the missing mass had transformed into energy (E)!
So when you think about how the ping pong balls were acting during the chain reaction, you can imagine how much energy there is to be harnessed.
When used in a nuclear power station, the heat energy produced by the reaction is used in the same way as most thermal power stations; it boils water, which produces steam, which turns a turbine that generates electricity!